Innovation & Advantages
-
High-throughput single exosome (100,000+) analysis
Simultaneous analysis of 100,000+ single exosome proteins in each sample allows you to discover low-abundance exosome subsets in your sample.
-
500+ multiplex protein detection
The multiplex immunoassay technology can be used to analyze the characteristics of 200+ protein components in a single exosome in real time, providing more comprehensive and accurate data analysis results.
-
<5 μl sample volume without purification
Most body fluids only require a small amount of samples, and there is no need for prior exosome isolation and purification steps, which further saves time and experimental costs.
-
Single-Signal molecular detection
High-sensitivity data results with detection resolution accurate to single exosome surface molecular proteins, which can identify low-abundance exosome surface proteins with higher disease diagnostic value.
-
Suitable for almost all body fluids and culture fluids
Such as plasma, urine, cell culture medium, and organ lavage fluid, which can be used for single exosome detection, and is suitable for a wide range of sample types, which greatly reduces the limitations of samples.
Proximity Barcoding Assay
The breakthrough single exosome detection technology has changed the dilemma that traditional exosome technology is often limited to total protein and total nucleic acid detection, and has realized high-precision and high-sensitivity exosome heterogeneity research.
Proximity Barcoding Assay (PBA) article: Wu et al. (2019) Profiling surface proteins on individual exosomes using a proximity barcoding assay

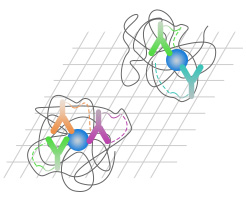
Revealing molecular co-localization at the nanoscale
Our Proximity Barcoding Assay (PBA) is a breakthrough technology designed to map molecular co-localization directly on nanoscale biological structures such as protein complexes, single extracellular vesicles (EVs), and organelles. By combining DNA barcoding with next-generation sequencing (NGS), PBA delivers single-structure resolution, multiplexed detection, and high-throughput insights, transforming how complex biological systems are studied.
How PBA Works
-
Step 1:
Capture Targets
Isolate and prepare the biological structures of interest.
-
Step 2:
Probe Binding
Multiplexed DNA-tagged antibodies detect proteins.
-
Step 3:
Proximity Barcoding
DNA barcodes record molecular neighborhoods.
-
Step 4:
Sequencing and decoding
NGS reconstructs nanoscale molecular profiles.
-
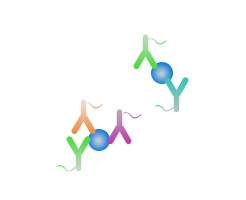 Multiplex antibody recognition
Multiplex antibody recognition
-
 Single exosome proximity barcoding
Single exosome proximity barcoding
-
 High-throughput sequencing
High-throughput sequencing
-
 Bioinformatics analysis
Bioinformatics analysis
Reference
-
Wu, Jicheng et al. “Tumor circulome in the liquid biopsies for cancer diagnosis and prognosis.” Theranostics vol. 10,104544-4556. 15 Mar. 2020, doi:10.7150/thno.40532
-
Kucuk, Nika et al. “Exosomes Engineering and Their Roles as Therapy Delivery Tools, Therapeutic Targets, and Biomarkers.” International journal of molecular sciences vol. 22,179543. 2 Sep. 2021, doi:10.3390/ijms22179543
-
Padmasekar, Manju et al. “Exposomes to Exosomes: Exosomes as Tools to Study Epigenetic Adaptive Mechanisms in High-Altitude Humans.” International journal of environmental research and public health vol. 18,16 8280. 5 Aug. 2021, doi:10.3390/ijerph18168280
-
Yang, Dexin et al. “Combined Analysis of Surface Protein Profile and microRNA Expression Profile of Exosomes Derived from Brain Microvascular Endothelial Cells in Early Cerebral Ischemia.” ACS omega vol. 6,34 22410-22421. 18 Aug. 2021, doi:10.1021/acsomega.1c03248
-
Pasetto, Laura et al. “Decoding distinctive features of plasma extracellular vesicles in amyotrophic lateral sclerosis.” Molecular neurodegeneration vol. 16,1 52. 10 Aug. 2021, doi:10.1186/s13024-021-00470-3
-
Paolillo, Mayra et al. “Fostering "Education": Do Extracellular Vesicles Exploit Their Own Delivery Code?” Cells vol. 10,71741. 9 Jul. 2021, doi:10.3390/cells10071741
-
Shan, Xiaoxiao et al. “The Biogenesis, Biological Functions, and Applications of Macrophage-Derived Exosomes.” Frontiers in molecular biosciences vol. 8 715461. 21 Jul. 2021, doi:10.3389/fmolb.2021.715461
-
Guo, Wei et al. “Single-Exosome Profiling Identifies ITGB3+ and ITGAM+ Exosome Subpopulations as Promising Early Diagnostic Biomarkers and Therapeutic Targets for Colorectal Cancer.” Research (Washington, D.C.) vol. 6 (2023): 0041. doi:10.34133/research.0041
-
Sun, Li, and David G Meckes Jr. “Multiplex protein profiling method for extracellular vesicle protein detection.” Scientific reports vol. 11,1 12477. 14 Jun. 2021, doi:10.1038/s41598-021-92012-6
Reference
-
Gao, Jing et al. “Exosomes Promote Pre-Metastatic Niche Formation in Gastric Cancer.” Frontiers in oncology vol. 11652378. 24 May. 2021, doi:10.3389/fonc.2021.652378
-
Prajapati, Sushant et al. “Biomimetic Nanotechnology: A Natural Path Forward for Tumor-Selective and Tumor-Specific NIR Activable Photonanomedicines.” Pharmaceutics vol. 13,6 786. 25 May. 2021, doi:10.3390/pharmaceutics13060786
-
Sun, Ruiting et al. “Proteomic profiling of single extracellular vesicles reveals colocalization of SARS-CoV-2 with a CD81/integrin-rich EV subpopulation in sputum from COVID-19 severe patients.” Frontiers in immunology vol. 14 1052141. 12 May. 2023, doi:10.3389/fimmu.2023.1052141
-
Kong, Lingnan et al. “Ascites-derived CDCP1+ extracellular vesicles subcluster as a novel biomarker and therapeutic target for ovarian cancer.” Frontiers in oncology vol. 13 1142755. 3 Jul. 2023, doi:10.3389/fonc.2023.1142755
-
Zhai, Ranran et al. “Genetic and phenotypic links between obesity and extracellular vesicles.” Human molecular genetics vol. 31,21 (2022): 3643-3651. doi:10.1093/hmg/ddac069
-
Min, Yuxin et al. “Single extracellular vesicle surface protein-based blood assay identifies potential biomarkers for detection and screening of five cancers.” Molecular oncology vol. 18,3 (2024): 743-761. doi:10.1002/1878-0261.13586
-
Wen, Yi et al. “Cell-derived nanovesicles prepared by membrane extrusion are good substitutes for natural extracellular vesicles.” Extracellular vesicle vol. 1 (2022): 100004. doi:10.1016/j.vesic.2022.100004